August 23, 2022, 10:59 AM
August 23, 2022, 10:59 AM
Among the typical symptoms of monkey poxdeclared a public health emergency of international interest by the World Health Organization (WHO) in July, include rashes and painful blisters on the skin, fever, muscle pain, swollen lymph glands and, rarely, could cause severe complications.
But while the vaccines used to treat smallpox might reduce the risk of getting the virus, currently there are no treatments that speed recovery in affected people. This was explained this Tuesday, in a virtual meeting, by the Oxford University expert Peter Horby, a specialist in emerging infections and director of the new Institute of Pandemic Sciences and leader of the trial; and Martin Landray, professor of medicine and epidemiology at the aforementioned academic center.
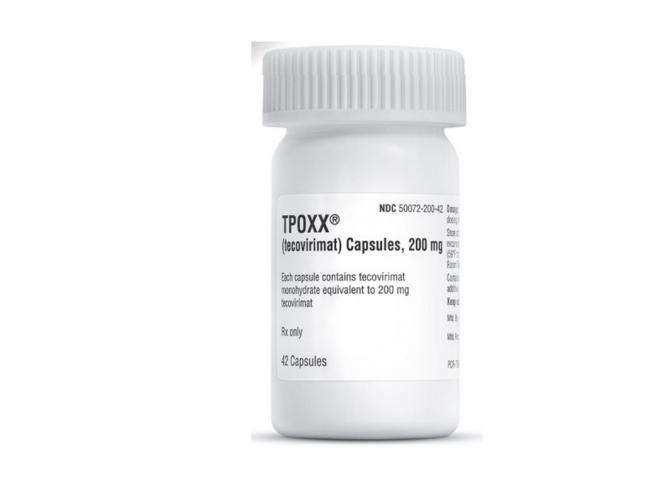
The new study, they stressed, will test the “efficacy and safety of Tecovirimat, an antiviral treatment initially developed for smallpox”.
The Tecovirimat prevents the virus from leaving infected cells, slowing its spread within the body. It is currently used to treat patients with severe monkeypox complications who have been hospitalized, but to date there are no trials to confirm whether this drug can help these patients recover from the disease.
At the press conference, Horby said that this country has experienced “a dramatic increase in cases” and pointed out that monkeypox is “a distressing infection, that sometimes causes quite severe symptoms, in which there are complications (…) and 10 percent of patients end up hospitalized”.
“Some of these complications are quite unpleasant, such as bacterial infections (…) and, rarely, death,” the expert recalled, while stressing the importance of finding a way to “stop transmission.”
With the new trial, with which they hope to recruit 500 volunteers, you want to check “if this drug is safe, if it’s effective and the goal is to find a treatment to help patients recover faster, reduce symptoms, and reduce complications.”
Participants will be randomly selected to receive either a 14-day course of 600 milligrams of Tcovirimat, given twice daily, or placebo treatment, and patients will undergo treatment from their homes.
To find out if these people recover faster on the drug, experts will assess the rate at which skin and mucosal lesions heal; how long it takes to test negative for the virus in samples taken from the throat and lesions and the time required by those volunteers who have to be hospitalized for complications will be controlled.