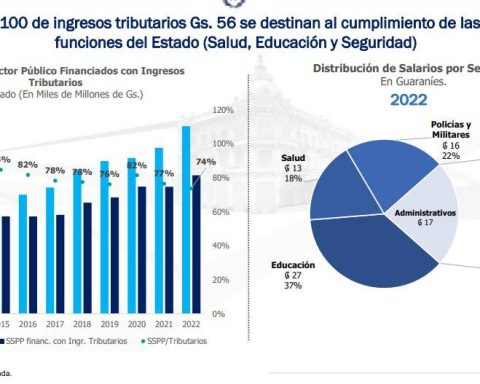
BioNTech achieved global recognition for its COVID-19 mRNA vaccine developed with partner Pfizer, but a significant part of the company’s product portfolio focuses on cancer therapy.
At the American Society of Clinical Oncology (ASCO) annual meeting this weekend, the company and Dr. Vinod Balachandran, of Memorial Sloan Kettering Cancer Center in New York, presented preliminary Phase I data on a new experimental vaccine ( BNT122) against pancreatic cancer.
The phase I trial is being carried out together with its partner Genentech, which belongs to the giant Roche. Specifically, they are studying the individualized mRNA-based neoantigen-specific immunotherapy (iNeST) autogen cevumeran (BNT122) in combination with Genentech’s Tecentriq (atezolizumab), an anti-PD-L1 immune checkpoint inhibitor, and chemotherapy. Patients in the study have successfully resected their pancreatic ductal adenocarcinoma (PDAC). That is, the injection worked to boost the immune response for some participants.
“Unlike other immunotherapies, these mRNA vaccines appear to have the ability to stimulate immune responses in pancreatic cancer patients. I am very excited about the development. Early results suggest that if you have an immune response, you may have a better outcome,” Balachandran explained. The trial came after researchers discovered that pancreatic cancer patients who are long-term survivors after surgery had a high number of immune cells that work to help fight the cancer.
The researchers recruited 19 patients who underwent surgery to remove the cancer and sent tumor samples to BioNTech in Germany. There, the team was able to formulate an individualized vaccine for each patient that was then administered intravenously, and the patients were also treated with immunotherapy. Vaccines work by using a tumor’s genetic code that teaches the body’s cells to create a protein to trigger an immune response; a technology that has already been used in Pfizer’s Covid vaccine.
Early data demonstrated a favorable safety profile and encouraging indications for clinical activity. That is why today BNT122 is being developed in multiple solid tumor indications. The data presented at the meeting of the 19 patients who underwent surgery, indicate that they received the drug atezolizumab. Of these, 16 received BNT122 9.4 weeks after surgery. Therapy was well tolerated, with only one developing vaccine-related Grade 3 fever and hypertension.
The therapy induced a de novo neoantigen-specific T-cell response in half of the patients from previously undetectable levels. And at an early median follow-up of 18 months, patients with the de novo immune response showed significantly longer recurrence-free survival (RFS) compared with patients without vaccine-induced immune responses.
“With only less than 5% of patients responding to current treatment options, PDAC is one of the cancers with the greatest unmet medical need,” said Dr. Özlem Türeci, MD, co-founder and chief medical officer of BioNTech. “We are committed to taking on this challenge by building on our long-standing research in cancer vaccinology and trying to break new ground in the treatment of such difficult-to-treat tumors. The results of this phase I study are encouraging. We look forward to further evaluating these early results in a larger randomized study,” he added.
iNeST immunotherapies are individualized cancer treatments that target a patient’s unique tumor. They contain unmodified, pharmacologically optimized mRNA encoding up to 20 patient-specific neoantigens that are identified through real-time next-generation sequencing and bioinformatic neoantigen discovery.
Neoantigens are proteins made by cancer cells that are different from proteins made by healthy cells. The mRNA is then inserted into the company’s proprietary intravenous RNA lipoplex delivery formulation.
Although the trial was small, Chris MacDonald, head of research at Pancreatic Cancer UK, said it was “really exciting to see this progress in a cancer that has been neglected for so long.” And he added that the vaccine could be a “vital new weapon against the deadliest common cancer.”
STUDY
In April, BioNTech presented data at the 2022 American Association for Cancer Research (AACR) conference describing another of its experimental cancer therapies, BNT211. It is a CAR-T cell therapy for patients with advanced solid tumors. BNT211 has a combination of two pharmaceuticals: an autologous CAR-T cell therapy targeting Claudin-6 oncofetal antigen (CLDN6) and a CLDN6-encoding CAR-T cell amplifying RNA vaccine (CARVac) using mRNA-technology. company’s lipoplex. The data presented was from 16 patients who received the therapy, some with CLDN6 CAR-T alone and others combined with CARVac.
In that study, indications included testicular cancer, ovarian cancer, endometrial cancer, fallopian tube cancer, sarcoma, and gastric cancer. Both groups demonstrated that the therapy was well tolerated and showed encouraging clinical activity. There were some side effects, including cytokine release syndrome, which was manageable, and mild indications of pancreatic toxicity.
Türeci, at the time, said: “Seeing early antitumor effects with even the lowest dose of CAR-T cells in this highly pretreated patient population is really remarkable and points to the potential of our CAR design and CARVac approach.”
The mRNA technology, which has been so effective as a vaccine against COVID-19, has not been largely tested in any other indication, such as cancer, although it has been hailed in the last decade as having great potential. BioNTech’s efforts so far are a good proof of concept for the technological approach to cancer. BioNTech also has programs for infectious diseases outside of COVID-19, including shingles, malaria, tuberculosis, HSV, and HIV.
It has at least five Phase II cancer programs and about a dozen Phase I for pancreatic cancer, non-small cell lung cancer, colorectal cancer, melanoma, HPV16+ head and neck cancer, prostate cancer, and other solid tumors .
Moderna, the other main competitor in mRNA therapies, is also developing mRNA therapies for autoimmune disorders and cancer, including a personalized cancer vaccine (mRNA-4157), a KRAS vaccine (mRNA-5671), a control (mRNA-4359) and other cancer treatments, as well as a broad program of infectious diseases.