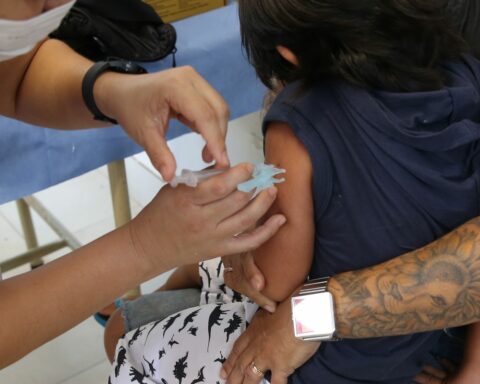
Despite being mandatory for newborns, the BCG vaccine – which protects against severe forms of tuberculosis – has recorded low rates of coverage. According to Datasus, from the Ministry of Health, the vaccine coverage of the immunizer dropped from 105%, in 2015, to 68.6% in 2021. BCG is part of the National Immunization Program (PNI) and is indicated to be applied immediately after the birth of the child.
According to Tania Petraglia, a member of the Scientific Department of Immunizations of the Brazilian Society of Pediatrics (SBP), the vaccine protects mainly against miliary tuberculosis, which occurs when the bacillus enters the bloodstream and reaches all organs, with a risk of meningitis. BCG also protects against other serious forms of tuberculosis, such as pulmonary tuberculosis.
Tania regretted the low coverage in the country and made an appeal to the population. “I make a call for the vaccination of all age groups from 0 to 100 years old and a little bit. Vaccines are a form of collective protection.”
In the expert’s assessment, there is a failure to guarantee this vaccination in primary care. “We need a more effective intervention from the municipal point of view”, she said.
intradermal
Vaccination with BCG is recommended from birth for children weighing 2 kg or more. As a single dose, BCG should be applied until the baby’s first month of life, because the incidence of the most severe forms of tuberculosis usually occurs while the child is still very young. There is no impediment, however, for individuals of any age to be vaccinated, although the degree of protection is lower.
According to Tania, BCG is an intradermal vaccine and, therefore, leaves a mark on the child’s arm. The other vaccines are intramuscular or intracutaneous, which are easier to apply.
Tania Petraglia informed that until very recently, when the vaccine did not cause a mark on the arm, revaccination was recommended. “Now, no need to repeat.” Just present the document proving the immunization.
Contraindications
Severe immunodeficiencies are contraindicated for vaccination, in addition to treatment by chemotherapy or radiotherapy and skin lesion at the application site. Likewise, HIV positive children should not take BCG. Already the children of carriers of this virus who have not been exposed to the disease can and should be vaccinated, indicated the pediatrician.
Factory
Today, July 1st, is BCG Vaccine Day. The name is derived from the Bacillus of Calmette Guérin (BCG), which was introduced in Brazil in the late 1920s, from a donation from the Pasteur Institute of Lille, France, where it was developed in 1921 by researchers Léon Calmette. and Alphonse Guérin.
BCG has been regularly used in the Brazilian population since the 1930s, produced in a factory owned by Fundação Ataulpho de Paiva (FAP).
The foundation, however, had its manufacturing and trading activities of pharmaceutical products interdicted in 2019 by the National Health Surveillance Agency (Anvisa) which considered that the factory posed a risk to health and was not in line with the standards of Good Practices of Health. Manufacturing (GMP).
For SBP, the BCG vaccine is very important for the health of children and adults and the Brazilian product is considered one of the best in the world. For Tania, with the closing of the Ataulpho de Paiva Foundation, it is time for Brazil to invest in an industrial park that meets the needs for manufacturing this immunizing agent in the country.
shortage
On May 29, several medical entities sent a letter to the Ministry of Health warning about the lack of BCG vaccine in the country’s health posts. The letter was signed by the SBP, the Brazilian Association of Collective Health (Abrasco), the Brazilian Tuberculosis Research Network (REDE-TB), the Brazilian Society of Immunizations (SBIm), the Brazilian Society of Pulmonology and Tisiology (SBPT) and the Brazilian Society of Medicine. Tropical (SBMT).
In response, the Ministry of Health informed, through the Health Surveillance Secretariat, that it hopes to normalize the distribution of vaccines from of September. At the moment, the average quantity available for each state is 500 thousand doses per month, against 1 million doses, previously made available.
According to the ministry, the BCG vaccine distribution quota for the states was reduced by 50% due to difficulties in the vaccine acquisition process – purchase, customs clearance and authorization by Anvisa for the product to enter the country – and unavailability quantity of doses in the national stock sufficient to maintain the quota that was usually being sent.
“The contingency and the reduction of the sending quota were necessary so that there was not a complete shortage of BCG vaccination services, to ensure, at least, the vaccination of children”.
anvisa
In a note, Anvisa confirmed to the Brazil Agency that the factory of Fundação Athaulpho de Paiva (FAP), located in the neighborhood of São Cristóvão (RJ), is paralyzed to carry out adjustments and corrections resulting from the last sanitary inspection.
“Manufacturing cannot resume until the necessary adjustments are completed and again the factory is inspected to verify the effectiveness of the corrections. The stoppage period depends on the time taken by the Foundation to carry out the adjustments. To schedule the inspection, FAP must inform the completion of activities and request the release inspection. That is, the deadline depends on the company responsible for the factory”.
THE Brazil Agency was unable to contact the Ataulpho de Paiva Foundation.