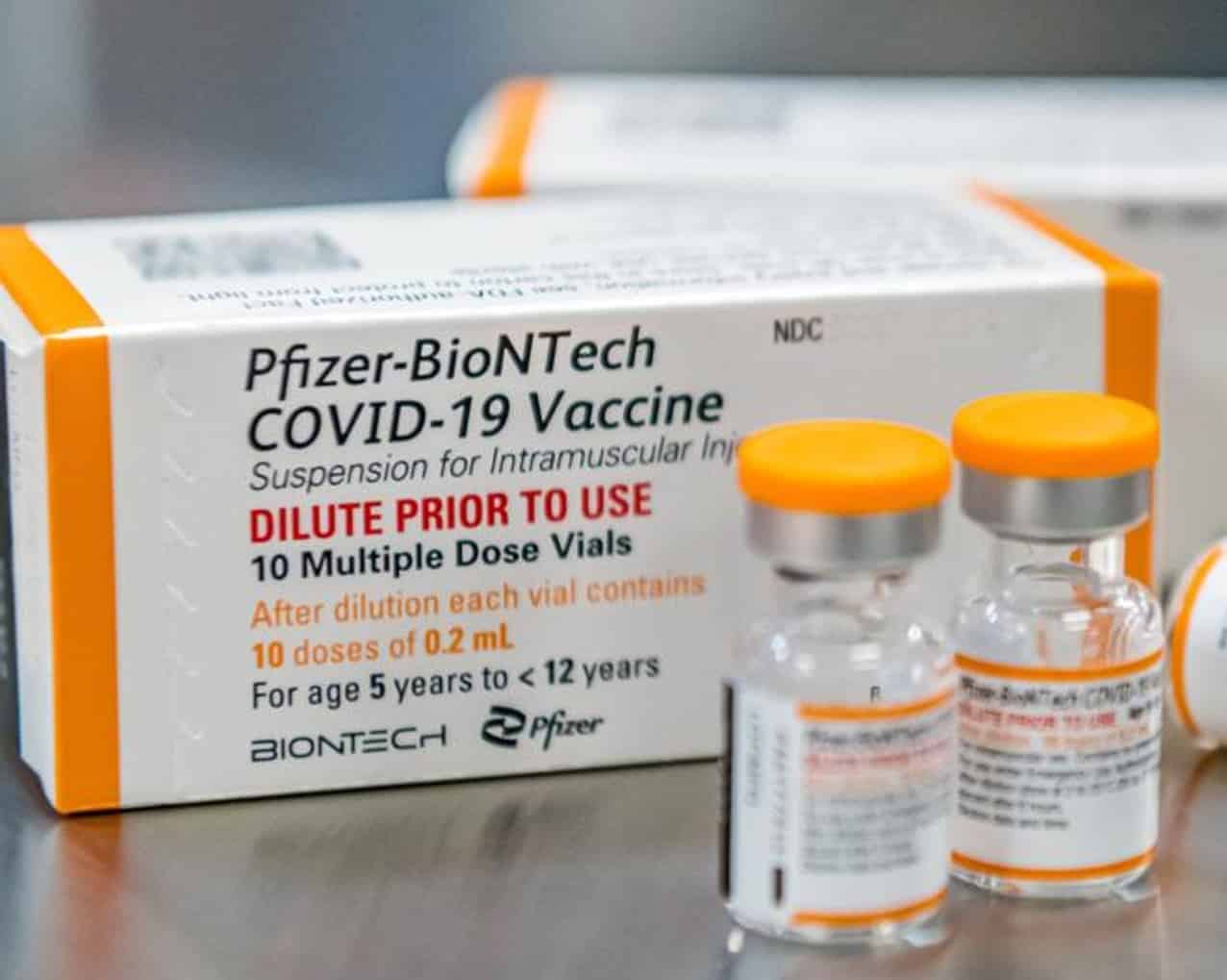Last Saturday, Nicaragua received a first batch with 650,660 doses of the Pfizer pediatric vaccine, which is part of a total donation of 1.4 million biologicals against covid-19 that the United States Government will deliver to the country, through the Covax Mechanism.
These vaccines will expand biologic options for children under 11 years of age who until now they were vaccinated with the vaccines: Soberana 02 from Cuba and Sinopharm of Chinese origin, The latter began to be applied to minors (three years and older) from the end of May 2022, without the Ministry of Health (Minsa) publicly informing it.
“I am proud to announce that thanks to the support of the United States, through USAID, 650,600 Pfizer pediatric vaccines against covid-19 will be available for boys and girls aged 5-11 years. Nicaraguan families will be able to protect the little ones in the house,” said the United States ambassador, Kevin Sullivan, on his Twitter account.
The diplomat also reported that a batch of 750,000 doses of this same vaccine will arrive in September, which will complete a total of 1.4 million doses. This week, the Minsa also received the donation of 1.2 million Pfizer vaccines for adults sent, separately, by the Government of Latvia and the European Union through the Covax Mechanism.
“We have expressed our appreciation to the Government and people of the United States and the Covax mechanism for this valuable contribution to the fight against the epidemic, and specifically against covid-19, a fight that we Nicaraguans have been waging day by day,” said Rosario Murillo, state spokesperson and vice president, on Monday.
Between February 23, 2021 and July 9, 2022, the Minsa has received 18,219,520 vaccines against covid-19 of ten types of vaccines, according to a monitoring CONFIDENTIAL. 52% of these correspond to donations and 48% to purchases made mainly from their political allies: Cuba and Russia, to whom the Ortega regime paid double for vaccines that still do not have WHO approval.
Pediatric Pfizer Features
The US Food and Drug Administration (FDA) authorized emergency use of the Pfizer-BioNTech vaccine to include children 5 through 11 years of age beginning October 29, 2021, and On June 17, 2022, it expanded the range of these vaccines for children older than six months.
As described by the FDA, Pfizer’s pediatric vaccines are given on a two-dose schedule given three weeks apart and at eight weeks the application of a third dose is recommended as a booster.
“The most commonly reported side effects among clinical trial participants—ages six to twenty-three months—who received the vaccine were irritability, decreased appetite, fever, and pain, tenderness, redness, and swelling at the injection site. These side effects were also reported in vaccine recipients two to four years of age, in addition to fever, headache, and chills,” the FDA details on its website.
The difference between the Pfizer vaccine for adults and that for children is that the dose is lower. Those over 12 years of age are given 30 micrograms, while those under 11 receive a dose of 10 micrograms. At the moment, the combination of these vaccines has not been approved, that is, the three doses must be of the same component.
Each bottle of Pfizer Pediatric contains ten doses, these must be used within six hours after the dilution of the vaccine. This type of biologicals are stored in ultra-freezers at -60 degrees and when thawed they must remain between +2 and +8 °C.
This vaccine is approved for use in children six months and older by the FDA. The WHO only approved it for children over five years of age.
Soberana 02 and Sinopharm are not approved
The other two vaccines that the Minsa applies to the pediatric population are Soberana 02, created by the Finlay Vaccine Institute (IFV) of Cuba, and Sinopharm, from the Institute of Biological Products in Beijing, China. Both biologicals are not approved by health agencies.
However, Sinopharm vaccines are given to children in several countries. In El Salvador, doses of this vaccine are applied to children between six and eleven years of age. In Argentina and Chile they also vaccinate children over three years of age.
The decision to apply this vaccine is due to phase one and two studies, published in the prestigious medical journal The Lancet, which supported the safety and immune response in groups of six and eleven years. However, the results of phase three, which are broader, are missing. In Nicaragua, the Minsa did not explain why it decided to apply this vaccine to minors.
According to the Ministry of Health, the pediatric population of Nicaragua amounts to 2.1 million. Of this sum, 1.3 million are children between the ages of two and eleven. The vaccination of these began on October 25, 2021, but it is unknown how many of these have already completed their vaccination schedule, how many received doses of Soberana 02 and how many are receiving Sinopharm.