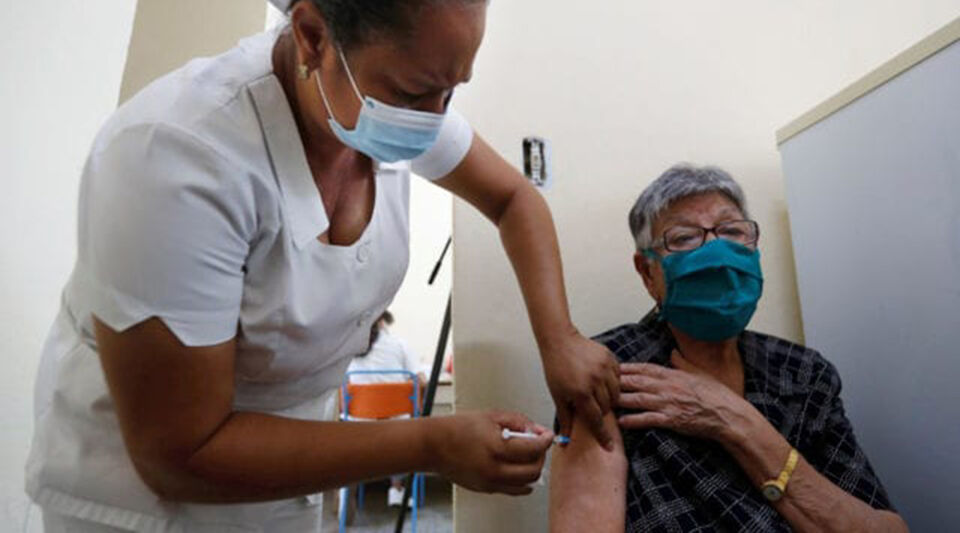
The WHO (World Health Organization) recognition process for Abdala, the Cuban vaccine against covid-19, continues to stop after the documentation was delivered to the health authorities in April. Eight months later, Eduardo Martínez Díaz, president of the BioCubaFarma business group, ensures a Granma that the problem is in the embargo, which causes the refusal of the banks to work with the Island.
The doctor explains to the Communist Party newspaper that “one of the components of the evaluation process is behind schedule.” BioCubaFarma announced months ago that it had decided to transfer the production of one of Abdala’s components to its new plant in the Mariel Special Development Zone (ZEDM). The WHO experts had planned to visit and assess the factory later this year, but this will not be possible.
“Although the production line, in which the formulation, filling and packaging operations of this complex are carried out, are already active and producing, the line in which the recombinant products are manufactured presents delays in its start-up,” he says. Martinez Diaz.
“We have been trying to make the payments for nine months, which have not materialized due to the refusal of several banks to carry out the transfer operation”
“This delay is due to the fact that it has not been possible to make payments to the company in charge of commissioning the equipment and systems for that production line. We have spent nine months trying to make the payments, which have not materialized due to the refusal of several banks to carry out the transfer operation”, he concludes. The doctor is hopeful that the study can be completed in 2023 and receive the endorsement of the health authorities, almost three years after the pandemic.
In the interview, the expert insists that the Cuban vaccines approved on the island –Abdala and Soberana 02 and Plus– are “safe, effective and capable of controlling the epidemic”, and for this he relies on the control of the disease in Cuba. as well as in the countries that have recognized them without waiting for the WHO.
According to Martínez, eight countries have given the authorization “and others are evaluating them”, although it has only been made public, to date, that Iran, Mexico and Nicaragua have given the green light to the Cuban serums. In the case of Tehran to Soberana, in whose preparation it is a partner through the Pasteur Institute. Mexico has also approved Abdala after receiving the approval of its regulator, the Federal Commission for the Protection against Sanitary Risks (Cofepris). In the case of Nicaraguathe two vaccines are likewise recognized.
In addition to the previous three, Venezuela, Vietnam or Belarus have inoculated some of the Cuban solutions after receiving batches exchanged or purchased from the Island. At the moment, the number of units delivered abroad and the money obtained through their sale are unknown. sale. The Cuban authorities announced that their vaccines would be cheap, although the Nicaraguan press estimated that they were 7 euros per unit based on a loan requested by the Government of Daniel Ortega from the World Bank, but there is no breakdown that allows verifying the concepts of the credit.
In any case, Cubans vaccinated with Abdala or Soberana –almost 100% of those immunized on the island– have had real difficulties traveling to many countries (including the US and the European Union) where it was mandatory to present a certificate or covid passport with one of the serums recognized by the WHO.
Martínez Díaz in his interview this Monday claims as a distinctive element of Cuban vaccines the high “thermostability” that require less cold than other vaccines (such as those from Pfizer or Moderna) and are stored at between 2 and 8 degrees, although, he says, they last up to a week at temperatures above 30 degrees, “which makes them attractive for use in poor countries, where there are difficulties in maintaining the cold chain of the immunogen,” he adds.
The slight side effects and the effectiveness that, they affirm, have been shown in the studies carried out on the Island, are also used by the official despite the fact that this would not be a differentiating characteristic from any other of those authorized. Martínez emphasizes that the results of the investigations have been published in various scientific journals, which requires that they have been subjected to peer review. “To date, more than 20 scientific articles have been published in high-impact journals and other reports continue to be prepared,” he says.
The doctor assures that Cuba, unlike other countries, has not suffered the onslaught of omicron, although it is difficult to verify, since it is necessary to sequence each case to find out, something that has not been done in a general way in any country in the world and much less on the Island, where mere diagnostic capacity was very limited at the time of the explosion of that strain. Martínez assured, in any case, that this situation is due to the fact that the vaccines developed on the Island decrease their capacity against this variant twice, while others do so 20 times.
“This phenomenon that we are observing has its explanation in the nature of the antigen that we use and the very design of our vaccines,” he explains. Martínez details that the island’s laboratories use the RBD antigen, which produces antibodies against a region of the protein that is common in all variants of the virus.
Meanwhile, other vaccines (those based on messenger RNA) have used the complete spike protein (S), which induces defenses against areas where there are mutations. The American Chemical Society warned at the end of 2021 of the possibility that protein S-based vaccines (Pfizer or Moderna) could fall short if the strategy was not diversified. Since then, the pharmaceutical They study the situation and possible improvements.
“We defend the hypothesis that vaccines based on the RBD antigen can constitute a universal booster for the rest of the vaccines against covid-19, amplifying protective immunity against the different variants of the Sars-Cov-2 virus, which have circulated or are could be generated in the future. Several groups of scientists around the world have recently published articles that support this hypothesis,” says Martínez.
________________________
Collaborate with our work:
The team of 14ymedio He is committed to doing serious journalism that reflects the reality of deep Cuba. Thank you for accompanying us on this long road. We invite you to continue supporting us, but this time becoming a member of our newspaper. Together we can continue transforming journalism in Cuba.