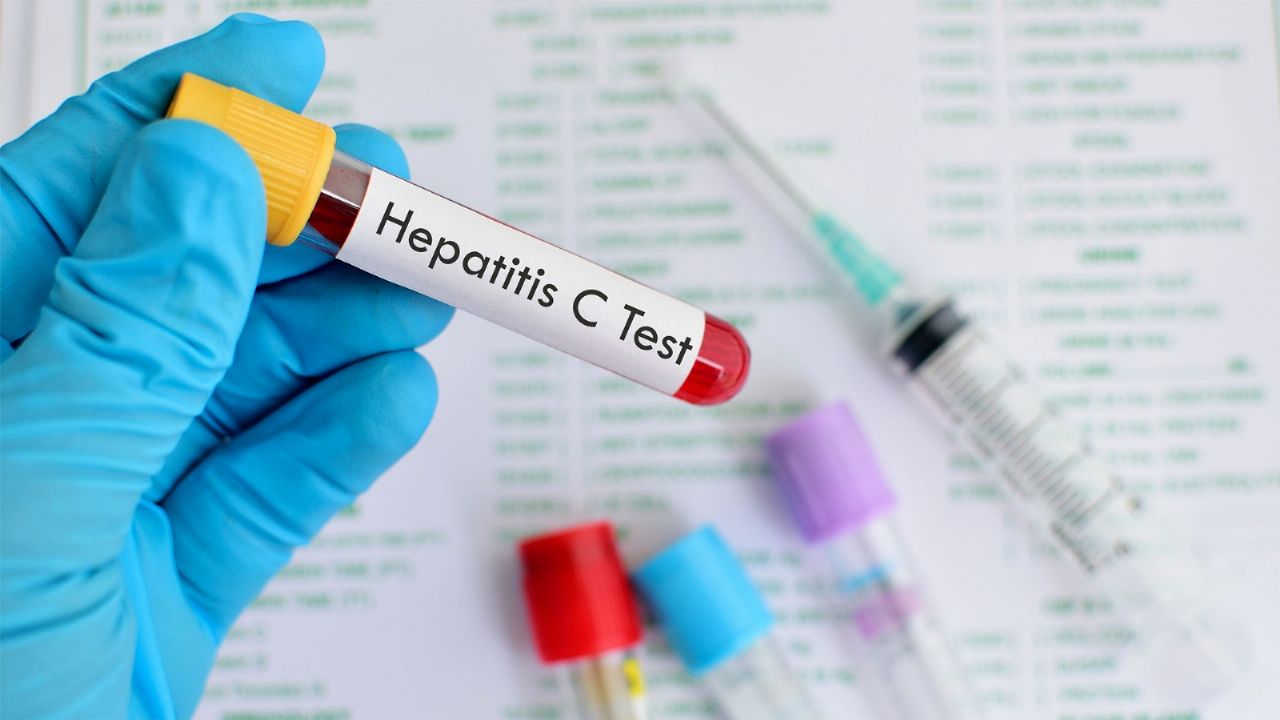
The National Administration of Drugs, Food and Medical Technology (ANMAT) approved a test that allows rapid detection of the hepatitis C virus (HCV).
The test that authorized the organism, it is also reliable, safe, and can be done in approximately 20 minutes, even less; which, according to ANMATwill allow an early and timely diagnosis of the disease.
The test that can now be used throughout the country, after the approval of the ANMAT, It is done through a finger prick with a small blood sample.; This process allows the identification of hepatitis C antibodies.
The new test has a sensitivity of 99.3% and a specificity of 100%, which proves to be very effective and will allow those suffering from this disease to receive timely treatment.

This, more so considering that 58 million people in the world suffer from an acute infection, which produces liver damage, as a result of the HCV virus.
Of these 58 million people, 332 thousand are in Argentina, so an early diagnosis also offers patients the possibility of having a better quality of life.

Even from the World Health Organization they advance several efforts to try to eradicate this disease before 2030, since more people are diagnosed every day.
This, given that the infectious picture generated by the virus is usually asymptomatic, so by not receiving timely care, the patient may have liver damage, cirrhosis, even cancer.

How is the test approved by the ANMAT
The test that was authorized by the organism and henceforth may be marketed throughout the national territory is Bioline™ HCV, produced by the Abbott laboratory.

The test will be for professional health use, so doctors can evaluate a patient from their own office and rule out whether or not they have the virus, and if necessary, order more tests to provide adequate treatment.