He Peru has joined international efforts for the development of a human immunodeficiency virus (HIV) vaccine. In the National University of San Marcos (UNMSM)a team of researchers carries out clinical trials and studies of preventive treatmentsas part of a global scientific network.
These investigations are carried out in the Technological, Biomedical and Environmental Research Center (CITBM)through his Clinical Trials Unit (UNIDEC)responsible for the management of clinical studies and biomedical research in the country.
Clinical trials in international coordination
One of the trials currently underway is focused on the HIV vaccine. The CITBM fulfills the function of reference laboratory for a global research network coordinated by the HIV Division of the US National Institute of Allergy and Infectious Diseases (NIAID).
In addition to HIV, the center has participated in research related to covid-19, monkeypox, zika, tuberculosis and hepatitis Cconsolidating its role in the study of infectious diseases.
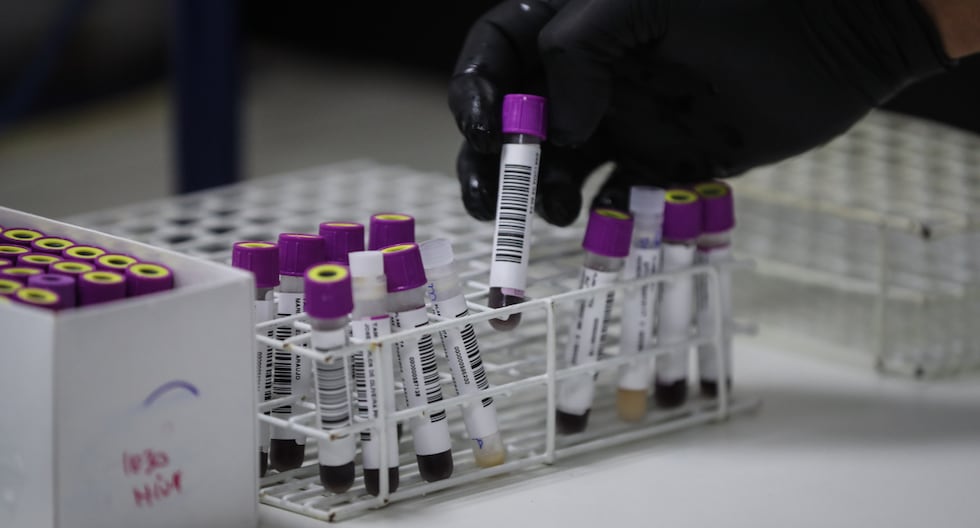
At UNIDEC, HIV screening and confirmation testingwhich include rapid, molecular and viral load tests, essential for evaluating participants in clinical studies.
Scientific rigor and safety of the studies
In interview with the Andean Agencythe doctor Leonela Candia, UNIDEC pathologistexplained that the trials follow a strict process from taking samples to sending them to the United States for specialized analysis.
“These clinical trials first seek to determine that the vaccine is safe to be able to distribute it worldwide and, furthermore, that it is effective in preventing new HIV infections,” he said.
The specialist highlighted that the CITBM has one of the few laboratories in the country certified to isolate polymorphonuclear cellsessential to evaluate the immune response generated by vaccines.
“These cells allow us to determine whether the immune response has been adequate. We isolate them and send them frozen to the United States for evaluation,” he explained.
Participant evaluation process
The UNIDEC laboratory applies a high-quality standardized protocolwhich begins with a screening to verify that volunteers meet the study criteria.
All participants provide their informed consentand various tests are performed to determine their HIV status, including:
- rapid tests
- Antigen and antibody tests
- Confirmatory tests (Genius)
- Molecular tests (Ginexper)
- Viral load testing
Subsequently, the samples are separated into plasma, serum and polymorphonuclear cellswhich are packaged, frozen and preserved until shipping.
Mosaic essay and regional cooperation
UNIDEC also participates in the trial called “Mosaic”aimed at measuring the vaccine efficacy in populations at higher risk of contracting HIV. In addition, it functions as a reference laboratory for research centers of Mexico and Argentinaprocessing samples from these countries.
A specialized software allows to ensure the complete traceability of each samplefrom its collection to its final processing. The data is statistically analyzed in the United States to determine the clinical effectiveness of the vaccine.
Advances, challenges and new trials
Dr. Candia recalled that in 2017 A large-scale clinical trial was developed that did not achieve the expected results after five years of research. However, the study allowed us to identify that the Vaccine molecule generated immunological response in CD4 and CD8 cellsalthough not enough.
That discovery gave rise to new clinical trialsamong them the HBTN206of which Peru is currently a part.
Clinical trials may be extended between two years in safety phases and up to five or six years in efficacy phaseswith annual evaluations of the data. According to the specialist, the success of these studies depends not only on the immune response, but also on sociodemographic and behavioral factors of the participants.