Angel Valdes | December 23, 2022
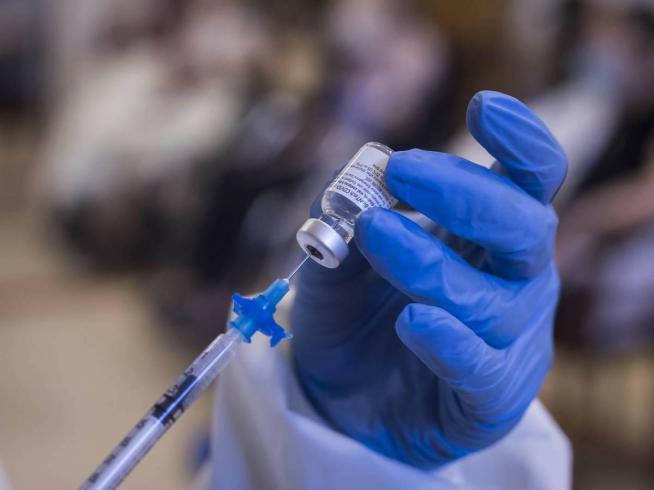
The Ministry of Health (Minsa) reported this Friday through the Directorate of Pharmacy and Drugs that it authorized the emergency use of the Pfizer-BioNTech Covid-19 bivalent vaccine for children aged 5 to 11 years.
According to the Ministry of Pharmacy and Drugs, the decision is based on the recognition of High Standard National Regulatory Authorities, Regional National Regulatory Authorities or the Emergency Use list of the World Health Organization (WHO).
“Pfizer bivalent COVID-19 vaccine is licensed for use in persons 5 to 11 years of age and older as a single booster dose administered at least 2 months after completion of a primary vaccination schedule with any monovalent COVID-19 vaccine and receive one of the most recent booster doses of any authorized monovalent vaccine,” stressed the Minsa.
The bivalent vaccine targets both the original strain of the coronavirus and the Omicron variant.