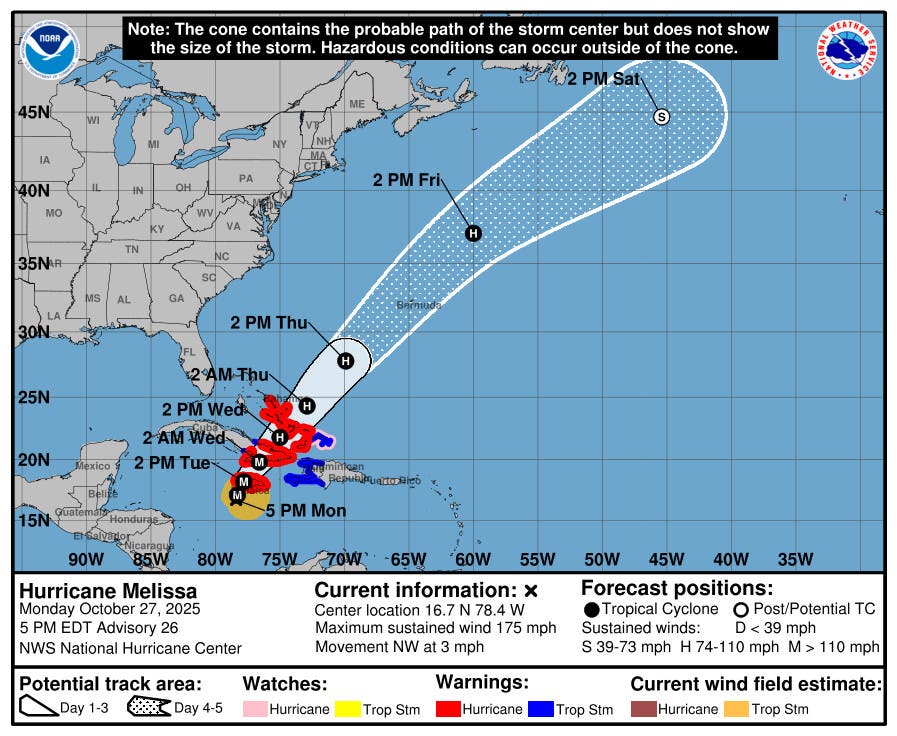
The Ministry of Health indicated that 99.8% of the drug Edetoxin was withdrawn after an outbreak of hospital-acquired infections linked to the Ralstonia pickettii bacteria was detected. The affected lot was completely immobilized, according to the Vice Minister of Public Health, Ricardo Peña.
The drug, intended only for use in hospital settings for the sedation of critically ill patients, is not prescribed on an outpatient basis. The authorities indicated that the situation is under control.
The affected batch corresponds to ABO25001 of Edetoxin 200 mcg/2 mL, manufactured in India and imported by Nordic Pharmaceutical Company SAC. The health alert was issued after 28 infected patients were registered in different hospital centers between August and September.
The first case occurred on October 22 at the San Borja National Institute of Children’s Health, where 13 infections were detected. In addition, cases were registered in three more hospitals, a private clinic and a dialysis center.
The bacteria Ralstonia pickettii It is an environmental microorganism of low virulence; However, its presence in the drug Edetoxin caused an epidemiological alert due to its use in hospitalized patients in critical condition. For this reason, Health Alert No. 116-2025 was issued to immediately immobilize the affected lot.
During quality controls, bacterial growth was identified in the sterility tests carried out on the contaminated batch.
The Minsa ordered the total immobilization of the contaminated lot. Of the 24,000 vials distributed, more than 84% were recovered during the first 40 hours, and subsequently 99.8% were removed, as confirmed by Vice Minister Peña to Canal N.
Likewise, the temporary closure of the importing laboratory was ordered due to deficiencies in manufacturing and traceability procedures. In addition, the health registration of the product was suspended and 64 additional registrations associated with the same laboratory are being evaluated.
It is worth mentioning that the Minsa indicated that three patients died in the context of the outbreak; However, he clarified that their deaths were due to causes related to their underlying illnesses and that a direct link to said medication has not been established.
Peña assured that the situation “is controlled” and that a causal link between the medication and the deaths has not been confirmed. It also reported that 15 of the affected patients were discharged.