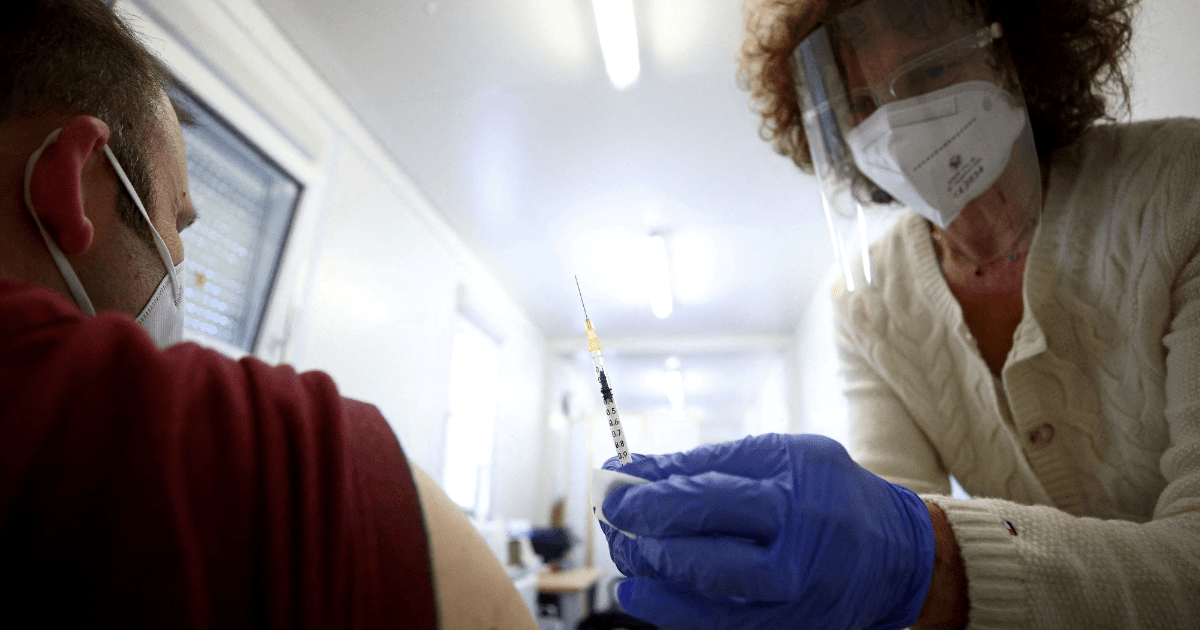
The European medicines regulator He said on Thursday that he hopes to have approved by September the vaccines adapted to face the coronavirus variantsWhat Omicron.
“Our priority is to ensure that the adapted vaccines are possibly approved by September at the latest in order to be ready for the rollout of the new immunization campaigns in the EU in the autumn,” he said. Marco Cavalieri, Head of Strategy for Biological Threats to Health and Vaccines at the European Medicines Agency (EMA).
“This would allow manufacturers to adjust their production lines accordingly.”
mRNA vaccines, manufactured by Pfizer-BioNTech Y modernare the most advanced, and clinical trials are ongoing.
It’s not yet clear whether the vaccines will be for one variant or two in one shot, Cavaleri added.