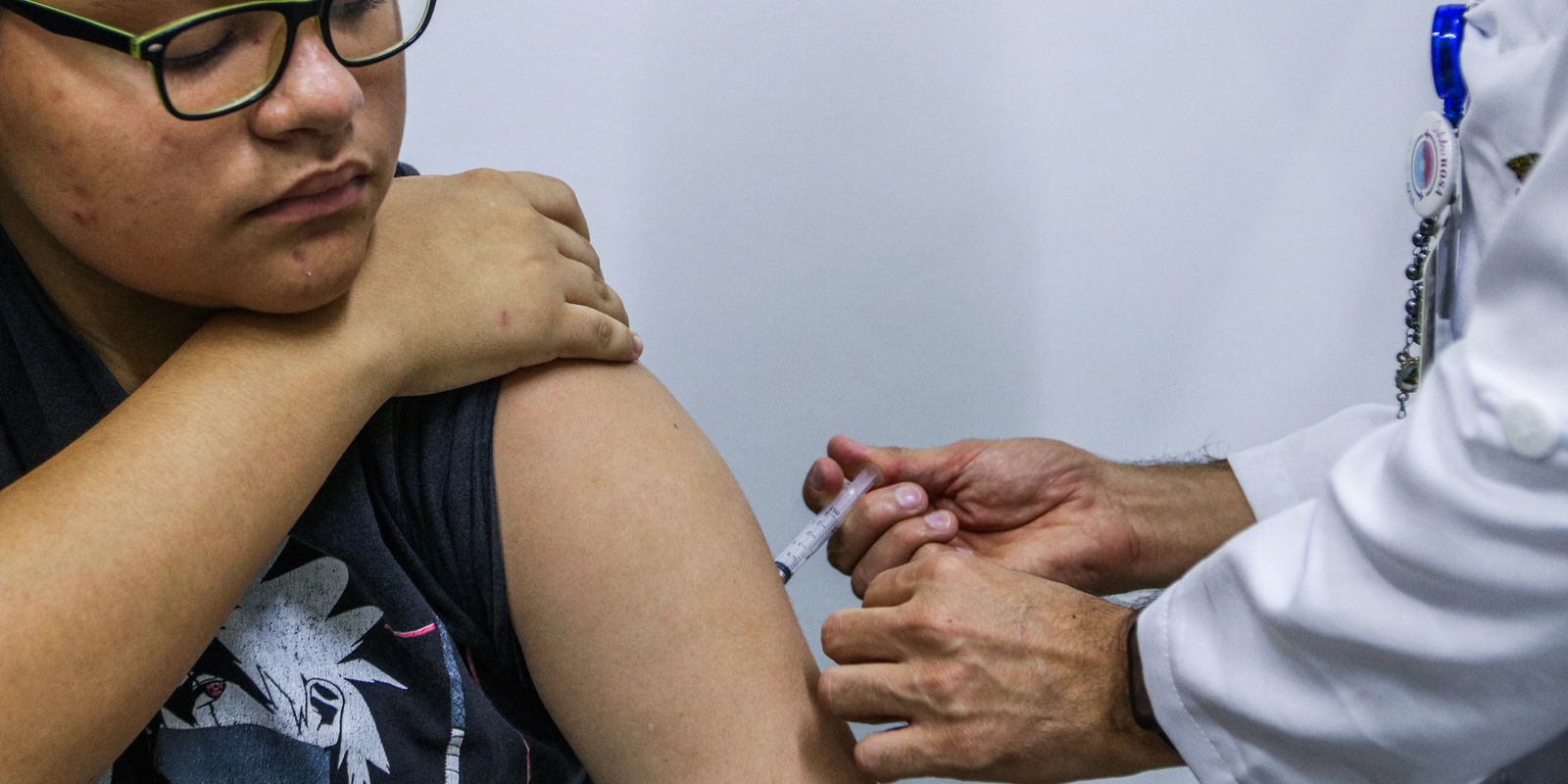
A year after the start of dengue vaccination in the Unified Health System (SUS), the demand for the immunizer in the country is well below expectations. From February 2024 to January 2025, 6,370,966 doses were distributed. The National Health Data Network, however, indicates that only 3,205,625 were applied to children and adolescents from 10 to 14 years, target group defined by the folder.
The age group, according to the ministry, concentrates the largest number of hospitalizations by dengue after elderly people, a group for which the immunizer Qdenga, by the Japanese pharmacist Takeda, was not released by the National Health Surveillance Agency (Anvisa). The vaccination scheme used by the folder consists of two doses with an interval of three months between them.
Understand
In January 2024, 521 municipalities were initially selected to start immunization against dengue in the public network as early as February. The cities made up 37 health regions considered endemic for the disease and met three criteria: large municipalities, with more than 100,000 inhabitants; High dengue transmission in the period 2023-2024; and greater predominance of serotype 2.
Currently, all federative units receive doses against dengue. The distribution criteria, defined by the National Council of Secretaries of Health (Conass) and the National Council of Municipal Health Secretariats (Conasems), follow recommendations of the Technical Advisory Chamber (CTAI). Health regions have been selected with large municipalities, high transmission in the last 10 years and/or high infection rates in recent months.
The definition of a target audience and priority regions, according to the Ministry, was necessary due to the limited capacity of doses supply by the manufacturer. The first shipment, for example, arrived in Brazil in January last year and had only about 757 thousand doses. The folder acquired all the amount provided by the manufacturer to 2024 – 5.2 million doses and contracted 9 million doses to 2025.
Priority for SUS
In a statement issued last year, Takeda informed the decision to prioritize the request of requests made by the Ministry for the provision of Qdenga doses. According to the note, the laboratory suspended the signing of direct contracts with states and municipalities and limited the supply of the vaccine in the private network, supplying only the necessary quantity for people who took the first dose to complete the vaccination scheme with the second dose.
“In line with the principle of equity in health, Takeda is committed to supporting health authorities, so their efforts are focused on meeting the Ministry of Health’s demand, according to the vaccination strategy defined by the National Immunization Program department that It considers age group and regions to receive the vaccine. As already announced, we have guaranteed the delivery of 6.6 million doses for the year 2024 and the provision of another 9 million doses for the year 2025. ”
Vaccine
The Qdenga vaccine was approved by Anvisa in March 2023. In practice, the process allows the commercialization of the product in Brazil, provided that the approved conditions are maintained. In December of the same year, the ministry announced the incorporation of the immunizer into the SUS.
In 2024, the immunizer was also prequalified by WHO. The entity defines Qdenga as an attenuated living vaccine that contains weakened versions of the four dengue virus serotypes and recommends that the dose be applied to children and adolescents from 6 to 16 years in places with high disease transmission.
“Prequalification is an important step in expanding global access to dengue vaccines, as it makes the dose eligible for the acquisition of UN agencies [Organização das Nações Unidas]including UNICEF [Fundo das Nações Unidas para a Infância] and PAHO [Organização Pan-Americana da Saúde]”, AVALIS, at the time, the director of regulation and pre-qualification of WHO, Rogerio Gaspar.
“With only two pre-qualified dengue vaccines so far, we hope that more vaccine developers will be presented for evaluation so that we can ensure that the doses will reach all the communities that need them,” he added. The other prequalified dose is that of Sanofi Pasteur.
Alert
Last month, the Brazilian Society of Immunizations (SBIM) issued a warning about the low demand for dengue vaccine. The entity stressed that the immunizer is currently available to a restricted group of people in 1,900 cities where the disease is most frequent and that only half of the doses distributed by the Ministry to states and municipalities was applied.
The warning follows recent prevention and monitoring actions of the Ministry of Health and comes at a time of concern because of dengue section 3 of dengue in various locations. The serotype, according to the ministry, has not predominantly circulated in the country since 2008 and, therefore, much of the population is susceptible to infection.
Sought by Brazil agencythe folder reported that the low availability to acquire Qdenga makes vaccination not the government’s main strategy against the disease. The ministry also highlighted the launch of the dengue and other arbovirus reduction plan, which provides for the intensification of the vector control of the Aedes aegyptimosquito transmitting the disease.
In early January 2025, the ministry again installed the Health Emergency Operations Center (COE), with the objective of expanding arbovirus monitoring in Brazil.
Numbers
In 2024, the country recorded the worst dengue epidemic, with 6,629,595 probable cases and 6,103 deaths because of the virus. By 2025, the Arbovirus monitoring panel already records 230,191 probable cases of the disease and 67 confirmed deaths, in addition to 278 deaths under investigation. The incidence coefficient at this time is 108 cases per 100,000 inhabitants.