The information began to circulate on Thursday and the president was consulted about it, but sent it to next Tuesday.
“INTERLOCUTOR: President, and nothing else to finish, Cofepris, if you can clarify that it is true that Cofepris had already authorized the use of the vaccine for children from 5 to 11 years of age since the beginning of March. Is it going to be announced soon or how was this authorization for the Pfizer vaccine?
“PRESIDENT ANDRES MANUEL LOPEZ OBRADOR: On Tuesday, if you think so, to have the information exactly,” the stenographic version refers.
In this regard, the Secretary of Health of Mexico City Oliva López was also consulted this Tuesday:
reporter: COFEPRIS endorsed the emergency use of the vaccine for children under 5 years of age and older, as long as the minors have protection; In this case, has there already been information from the Federal Government for the Government of Mexico City on the vaccination of children under 5 years of age and older?
Oliva López: We are attending, as I have pointed out on other occasions, the amparos; when there is an order from the judge, it is served. We know that COFEPRIS has already approved the pediatric presentation, but it is not yet available because it is a purchase by the Government of Mexico and that it would have to provide us; So, we are aware of this situation.
When was the vaccine approved for minors?
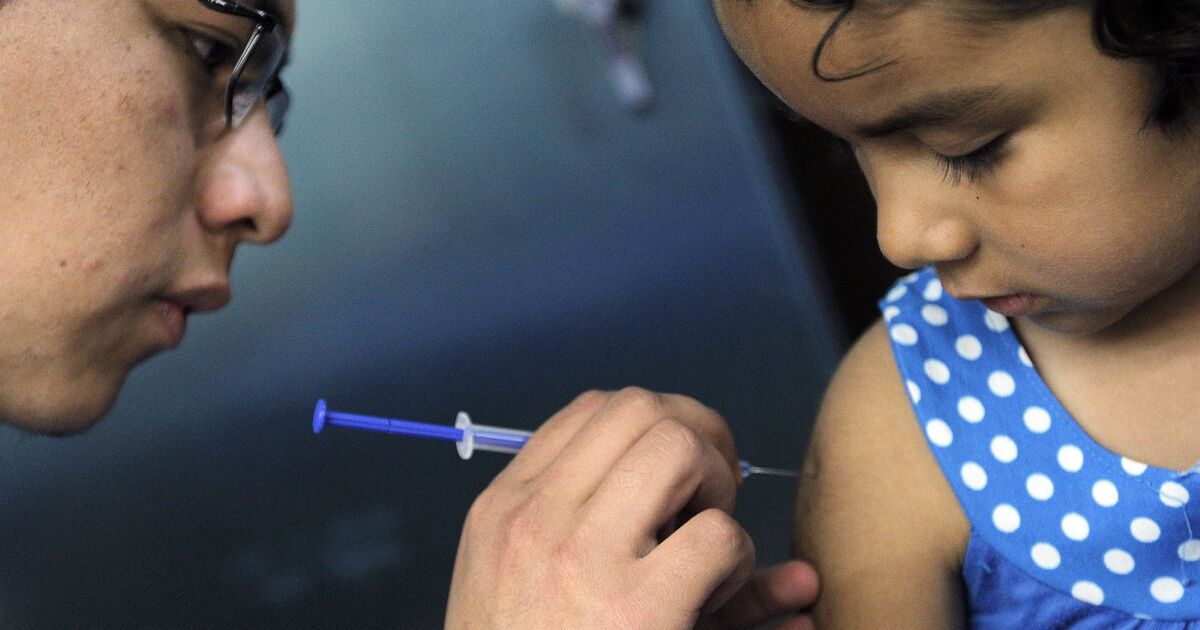
On March 3, Cofepris updated the list of vaccines that have obtained authorization for emergency use.
This publication includes the group from 5 to 11 years old, for whom the Pfizer brand of vaccine is contemplated. In addition, the form in which the dose will be applied is indicated.
But the inclusion of children between 5 and 11 years of age in the national health system was not reported through any official communication or publication on social networks.
It should be remembered that parents of relatives have filed several amparos against the refusal of the Ministry of Health to vaccinate their minor children against COVID-19.