What is Molnupiravir? The pill for the treatment against covid-19 that the permission from the General Directorate of Medicines, Supplies and Drugs (Digemid) to be marketed in Peru, yes, under a prescription, but what are its benefits? how is it administered?; And why is the Ministry of Health reluctant to make it part of the treatment protocol in the country?
MORE INFORMATION: These are the side effects that vaccines against COVID-19 can cause
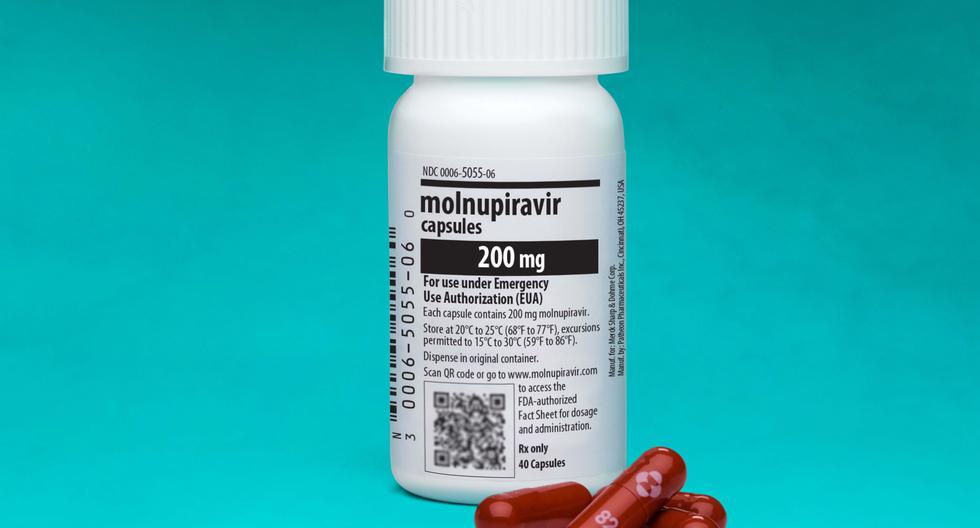
On February 17, 2022, Digemid granted the Conditional Sanitary Registration to Molnupiravir, a drug that has been developed by the pharmaceutical company Merck Sharp & Dohme (MSD), which ensures that the pill has demonstrated efficacy and safety in reducing hospitalizations and deaths. by coronavirus.
This drug has also been approved by the FDA and the European Medicines Agency (EMA), but the Ministry of Health considers that there is not enough evidence for its use in the country. Pay attention to the note.
WHAT IS MOLNUPIIRAVIR?
Molnupiravir It is a drug that reduces hospitalizations and deaths from coronavirus in 3 out of 10 people, that is, it has an efficacy of 30%. The drug was authorized in 2021 by the FDA and the European Medicines Agency (EMA),for the treatment of mild to moderate COVID-19 in adults who have serious illnesses or are at risk of dying for their age, including asthma and obesity.
The FDA clarified in a statement that the pill can only be administered to those who cannot access other treatments endorsed by the US authorities or who are clinically considered to be better off taking the treatment, known in the rest of the world as MSD.
HOW DOES THE DRUG AGAINST COVID-19, MOLNUPIRAVIR, WORK?
Molnupiravir is a drug that works by introducing errors into the genetic code of the virus SARS-CoV-2, which prevents the virus from replicating and kills it.
“This medicine has a mode of use that is very interesting: it is called error catastrophe. When the medicine is taken this medicine manages to penetrate the cells and manages to enter where the coronavirus is. When the coronavirus comes into contact with this drug and begins to replicate, the drug causes so many errors in its replication that it collapses, destroys it, because in that realization of the virus so many mutations are made that the virus can no longer replicate effectively and, therefore, the disease decreases”explained Dr. Elmer Huertas in RPP.
HOW DO YOU TAKE THE COVID-19 DRUG, MOLNUPIRAVIR?
Molnupiravir is administered as follows:
- Four 200-milligram capsules orally every 12 hours for five days. That is, a total of 40 pills must be consumed.
Note: It is not authorized for use for more than five consecutive days.
WHO CANNOT TAKE MOLNUPIIRAVIR, THE DRUG AGAINST COVID-19?
- The drug is not authorized for people under 18 years of age because, according to the FDA, it can affect the growth of bones and cartilage.
- It cannot be given to pregnant women because if the action of the drug reaches an embryo, which is in formation, it can cause congenital malformations. If it is to be used by women of reproductive age, they should take a pregnancy test.
- Not licensed for pre- or post-exposure prevention of COVID-19
- It is not approved for treatment initiation in patients hospitalized for COVID-19 because no treatment benefit has been observed in people when treatment is initiated after hospitalization for COVID-19.
sexual intercourse According to the FDA, men of reproductive age who are sexually active with women of childbearing age are recommended to use reliable contraception correctly and consistently during treatment with molnupiravir and for at least three months after the final dose. .
WHY IS THE MINSA NOT IN FAVOR OF MOLNUPIIRAVIR, THE DRUG AGAINST COVID-19?
The General Director of Strategic Interventions of the Ministry of Health (minsa), Alexis Holguín, clarified that although the drug Molnupiravir has received conditional health registration from Digemid, this does not mean that it will be supplied to hospitals in Peru as part of the treatment against COVID-19.
The official explained that this developed drug is not approved in the treatment protocol in hospitals in the country, because it is considered that there is not much scientific evidence on its use. “This drug was tested in unvaccinated with a different variant and an initial efficacy of 50% and after its approval, a study came out reporting that it was 30%. When we did the review with the National Institute of Health (INS), the study group that had studied this drug was very small. So there is not enough information.he explained.
Holguín, explained that although the drug has the green light for Digemid, it is part of a “administrative process” and it does not mean that it will be for sale. In this sense, he recalled that, in November 2021, the aforementioned regulatory entity also approved the health registration of another drug against COVID-19, but to date it has not been used in the country nor is it marketed.