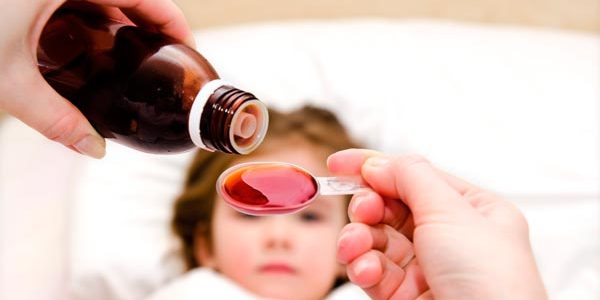
In the past four months, several incidents of over-the-counter cough syrups for children with “confirmed or suspected contamination with high levels of diethylene glycol and ethylene glycol” have been reported to the World Health Organization (WHO) by some countries. been published by the Ministry of Health (Minsa) who states that none of these drugs are registered in Panama.
The Minsa indicated that these cases are from at least seven countries, associated with more than 300 deaths in three of these countries, mostly children under five years of age, due to the fact that these contaminants are toxic chemical substances that are used as industrial solvents and antifreeze agents. they can be deadly even in small amounts and should never be found in medicines.
Through the National Directorate of Pharmacy and Drugs of Minsa, it was reported that the World Health Organization (WHO) urged the health authorities to reinforce measures to prevent, detect and respond to incidents with falsified and substandard quality medical products. In children.
The WHO has issued three global medical alerts addressing these incidents. On October 5, 2022, I report an outbreak in The Gambia; on November 6 in Indonesia and on January 11, 2023 in Uzbekistan.
At the same time, he urged regulators and the government to detect and withdraw from circulation in their respective markets any inferior quality medical product that has been identified in the WHO medical alerts mentioned as possible causes of death and disease.
On the other hand, it requests manufacturers to control the purchase of excipients from qualified suppliers. To do this, it insists on extensive testing upon receipt of supplies and prior to their use in the manufacture of finished products.
Regarding distribution, the WHO urges you to always verify signs of counterfeiting of medicines and other health products that you distribute and/or sell. In addition, it requires the distribution and/or sale, only, of medicines approved by the competent authorities.
To this end, it calls for the maintenance of accurate, complete and adequate records, and the hiring of qualified personnel for the handling of medicines, without forgetting, advising the public on the proper use of medicines.
The National Directorate of Pharmacy and Drugs of the Minsa reiterates that the products contained in the three world medical alerts are not registered in Panama.
It also added that the Panamanian pharmaceutical regulation requires the presentation of negativity of the contaminating compounds ethylene glycol and diethylene glycol, for the importation of each batch of oral liquid products that contain in their formulation the excipients of glycerin, sorbitol, propylene glycol or batch of these materials that used in the manufacture of pharmaceuticals.