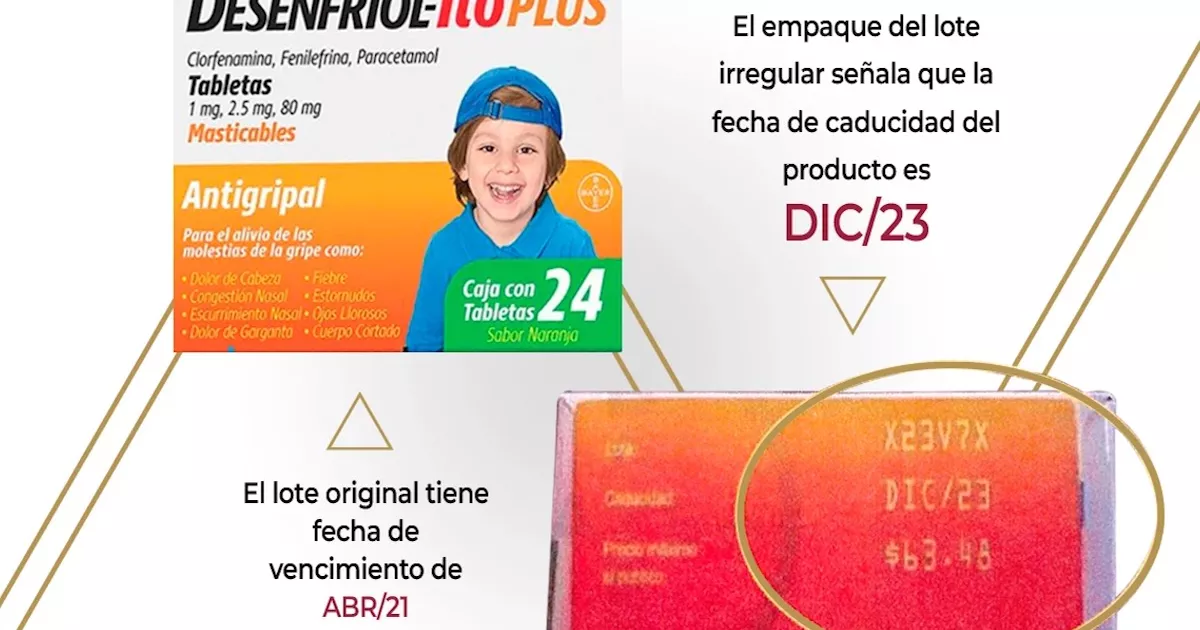
As for the drug Desenfriol-ito, the agency detailed that its presentation in chewable tablets was falsified, with the name X23V7X and expiration date of December 2023.
Bayer de México, the pharmaceutical company that produces this medicine, noticed the counterfeiting when it detected that they changed the expiration date, since the original corresponds to April 2021, Cofepris detailed.
Another characteristic of counterfeit tablets is that the image on the packaging does not correspond to the authentic one of that batch.
For this reason, the health authority urged pharmacies and distributors to review the inventory of Desenfriol-ito Plus and, in case of having parts of the counterfeit product, suspend its sale and notify the agency.
“Cofepris recommends that, prior to purchasing this treatment, it be verified that the batch, expiration date and image correspond to those authorized and that the counterfeits cited here be avoided,” he explained in a statement.
And they also counterfeit Rosel medication
Regarding the medicine Rosel Solution, which contains amantadine, chlorphenamine and paracetamol, Cofepris explained that the counterfeit is the 60 ml solution, with batch 200413 and an expiration date of December 2024.