Eight out of ten Brazilians have already taken two doses or a single dose of vaccines against covid-19 and just over half of Brazilians have already received at least the first booster dose. With so many people immunized, mortality from the disease continues to fall, but researchers continue to work not to lose the race against the genetic evolution of the coronavirus and continue to strengthen the population’s immunity in the future. This is the case of the CT Vacinas team at the Federal University of Minas Gerais (UFMG) and the Oswaldo Cruz Foundation (Fiocruz), which is currently gathering the latest documents so that a 100% national vaccine project has the human trials started in 2023.
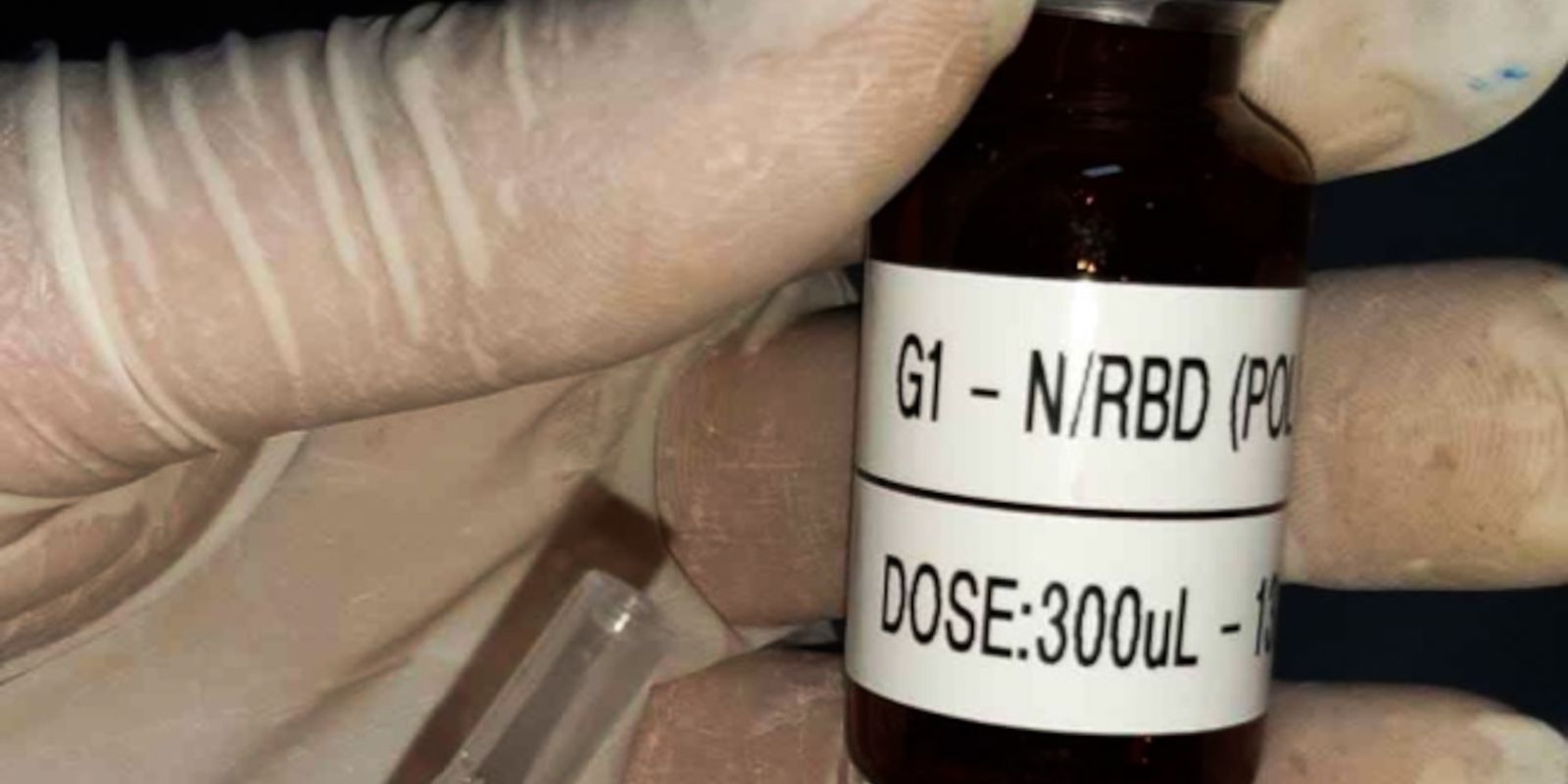
SpiN-TEC, as the mining vaccine is called, began to be developed in 2020, when variants were not yet a concern. Since then, the epidemiological scenario has changed several times, with waves of cases caused by the new versions of SARS-CoV-2, increasingly transmissible by mutations associated with the Spike protein – also called the S protein-, the virus’ main weapon for invade human cells.
Coordinator of the team that develops the vaccine, Ricardo Gazzinelli, explains that, if the studies prove the effectiveness of SpiN-TEC, it should join the team of vaccines of Monday generation, already calibrated to prevent a virus that evolved after more than two years of contagion.
“What are you calling vaccines for Monday generation are vaccines that would have a broader spectrum of action”, he says, who describes that this is due to the use of the S protein of the ancestral coronavirus and the Ômicron variant in the same vaccine, so that antibodies are created that react to both. ” This is an issue that regulatory agencies will start demanding from one hour. The problem is, if when the vaccine comes out, there is already a new variant.”
Protein S is the traditional target of vaccines for two important points: it triggers an immune reaction and is the tool for invading human cells. Despite this, it accumulates a large number of mutations, making it difficult for the antibodies to work. Therefore, the vaccine update bets on the combination of a new S protein with the ancestral S protein in the formulation of vaccines.
The researcher argues that, in this sense, the SpiN-TEC project is interesting, as it combines the S and N proteins of the coronavirus. Unlike S, the N protein is more stable and also triggers a reaction of T lymphocytes, another defense mechanism of the human body, which, in theory, will give less chance of escape to current and future variants.
These questions remain important because the scientific community is still unable to determine what the need for boosters will be, nor for whom they will be needed in the future. Thus, the researcher adds that SpiN-TEC could be produced in partnership with public research institutes, such as Bio-Manguinhos and Butantan, or with private companies, and its technological platform has logistical facilities.
“It is a very stable vaccine. It lasts two weeks at room temperature and six months in the refrigerator, which makes distribution much easier. Even more so in Brazil, which has such a large extension and areas that do not have such a good infrastructure”, he says. he. “The protein is a recombinant protein produced in bacteria, a very classic model of protein production, a cheap model. It is an existing infrastructure in Brazil”.
Before reaching the National Immunization Program, however, it is necessary to prove that the vaccine works. Tests carried out on animals have already demonstrated the ability to control the viral load and symptoms of covid-19, but tests in humans must be started, with authorization from the National Health Surveillance Agency. The agency has been guiding the researchers in relation to its requirements, if everything is aligned, the clinical trials begin at the beginning of next year, and can be completed in less than a year.
Testing the effectiveness of a vaccine that will be used as a booster in an already vaccinated population requires different protocols than testing a proposed vaccine as the first contact of a population against an antigen. Gazzinelli explains that, for this reason, the clinical trials of SpiN-TEC can be even faster than those of vaccines that have to wait a while until a certain number of volunteers get sick so that the placebo group can be compared to the vaccinated.
“It will be evaluated by immunological markers. If it induces a strong immune response against the virus, that will be an important selection criterion to allow the vaccine to advance. The studies are being designed in this way, to design an immunological marker to evaluate effectiveness”, he explained, who added that, in this case, the vaccine will need to be equal to or superior to the immunizers that are already on the market.