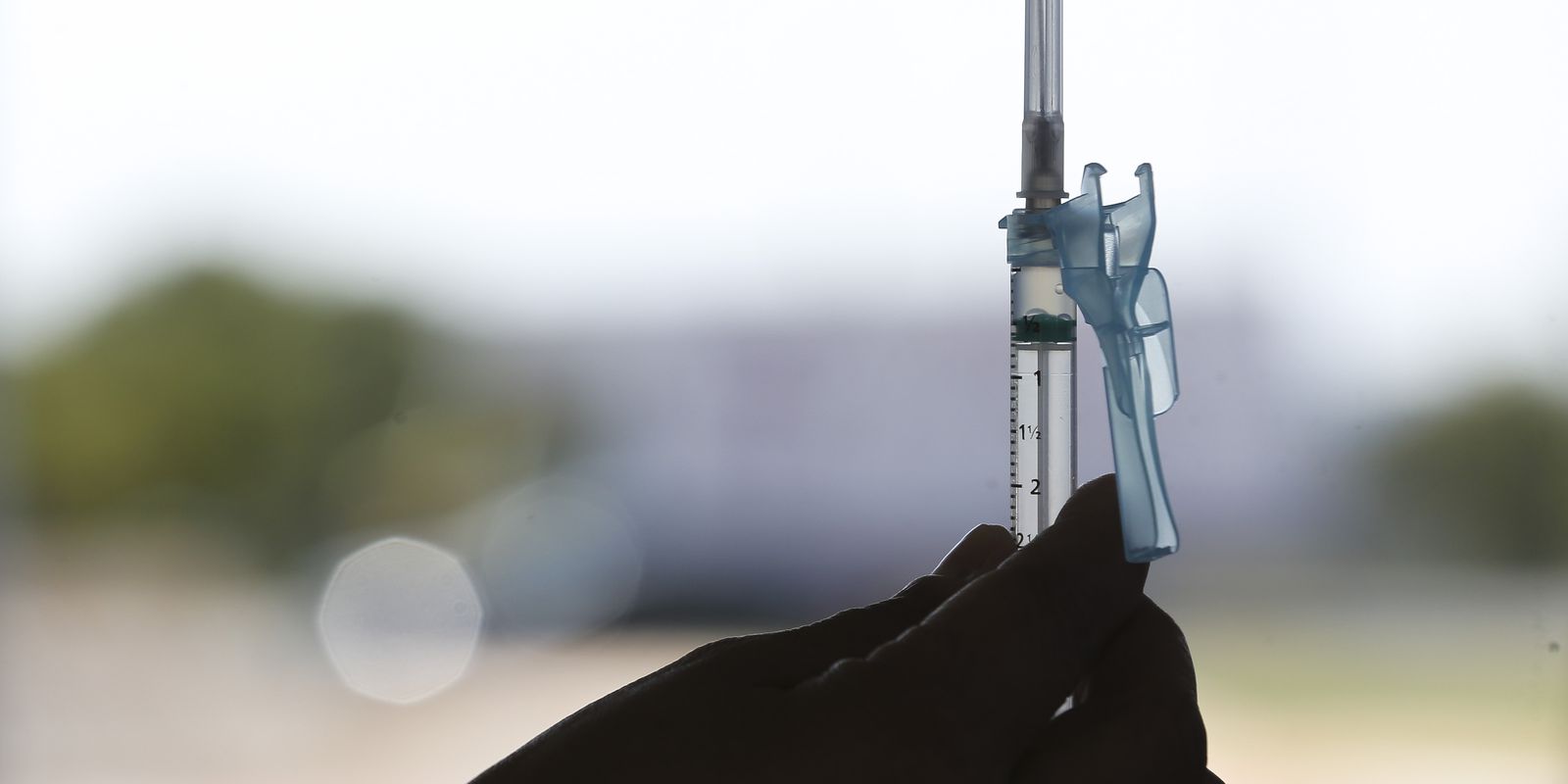
The Minister of Science, Technology and Innovation, Marcos Pontes, said today (1st) that the Brazilian vaccine against the new coronavirus should be available for use in the population in nine months. Called RNA MCTI CIMATEC HDT, the immunizer against covid-19, began phase 1 testing in patients in January.
The immunizer, developed by Brazilian researchers from the MCTI Virus Network in partnership with the American HDT Bio Corp, is financed by the Federal Government, through the Ministry of Science, Technology and Innovation (MCTI), and is produced at SENAI CIMATEC in Salvador (BA). ).
Phase 1 tests, authorized by the National Health Surveillance Agency (Anvisa) and the National Research Ethics Commission (Conep) are also being carried out in Salvador.
“We invested in 16 vaccine technologies in Brazil. Of these 16, five entered Anvisa to start clinical trials. One of them passed, was approved by Anvisa and the clinical trials have already started, which should last nine months”, said the minister who participated in the Mobile World Congress 2022, the main fair in the world of the telecommunications sector, held in Barcelona.
The vaccine uses messenger RNA technology. In this type of vaccine, the genetic code of the virus goes inside the body, and there, they provide instructions for the cells and immune system to build a response and generate antibodies.
RepRNA technology allows RNA to be able to self-replicate within cells, which ensures a robust and long-lasting immune response with a lower dose of vaccine. According to Pontes, the investment applied by the government for the elaboration of the immunizing agent will be R$ 350 million.
The phase 1 trial foresees the participation of 90 healthy adults, aged between 18 and 55 years and aims to assess the safety, immunogenicity (ability to generate an immune response), and reactogenicity (possible adverse reaction in the body) of the vaccine.
The test schedule provides for the application of the immunizer in two doses at different intervals. The first group will receive two doses 29 days apart; the second group will receive two doses 57 days apart. A third group of volunteers will receive a single dose of the vaccine. In addition, three dose levels of 1 μg (microgram), 5 μg or 25 μg will also be evaluated.
Tests with volunteers should also take place in the US and India.