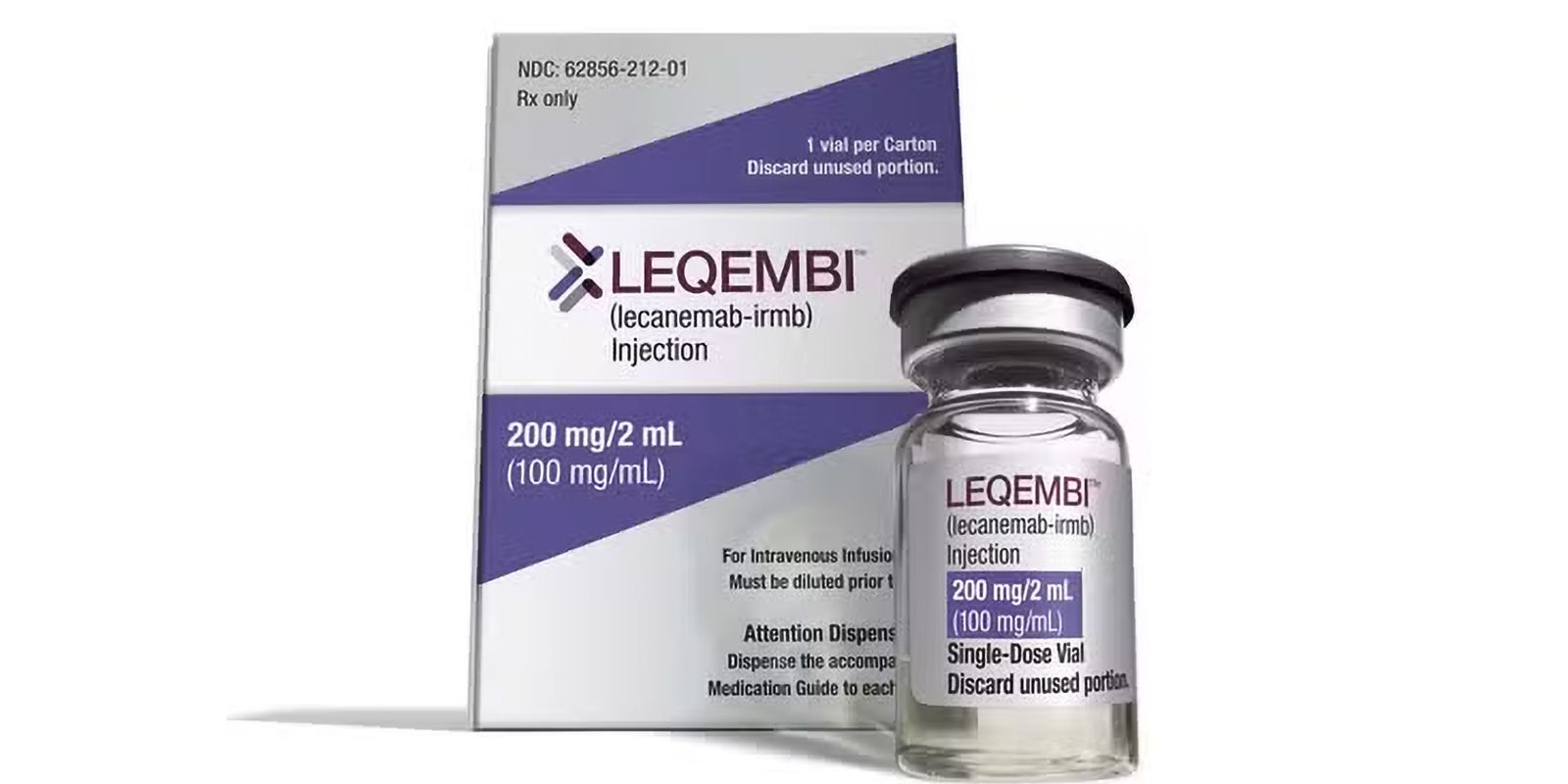
The National Health Surveillance Agency (Anvisa) released a new medicine, Leqembi, for the treatment of patients diagnosed in the early stages of Alzheimer’s disease. The approval was published in the Official Gazette of the Union on the 22nd of last month.
The medicine, produced with the antibody lecanemab, is indicated to delay the cognitive decline of people who already have mild dementia caused by the disease.
According to the Anvisa registry, lecanemab reduces beta-amyloid plaques in the brain. The accumulation of these plaques is a defining feature of Alzheimer’s disease. The product is a solution for dilution for infusion.
Study
Anvisa announced that the drug’s clinical effectiveness was evaluated in a main study that involved 1,795 people with early-stage Alzheimer’s disease, who had beta-amyloid plaques in the brain and received Leqembi or placebo.
“The main measure of effectiveness was the change in symptoms after 18 months”, pointed out Anvisa. The assessment took place using a dementia scale called CDR-SB, used to test the severity of Alzheimer’s disease in patients.
The scale includes questions that help determine how much the patient’s daily life has been affected by cognitive impairment. According to the study, in the subgroup of 1,521 people, patients treated with the new drug showed a smaller increase in CDR-SB score than those who received placebo.