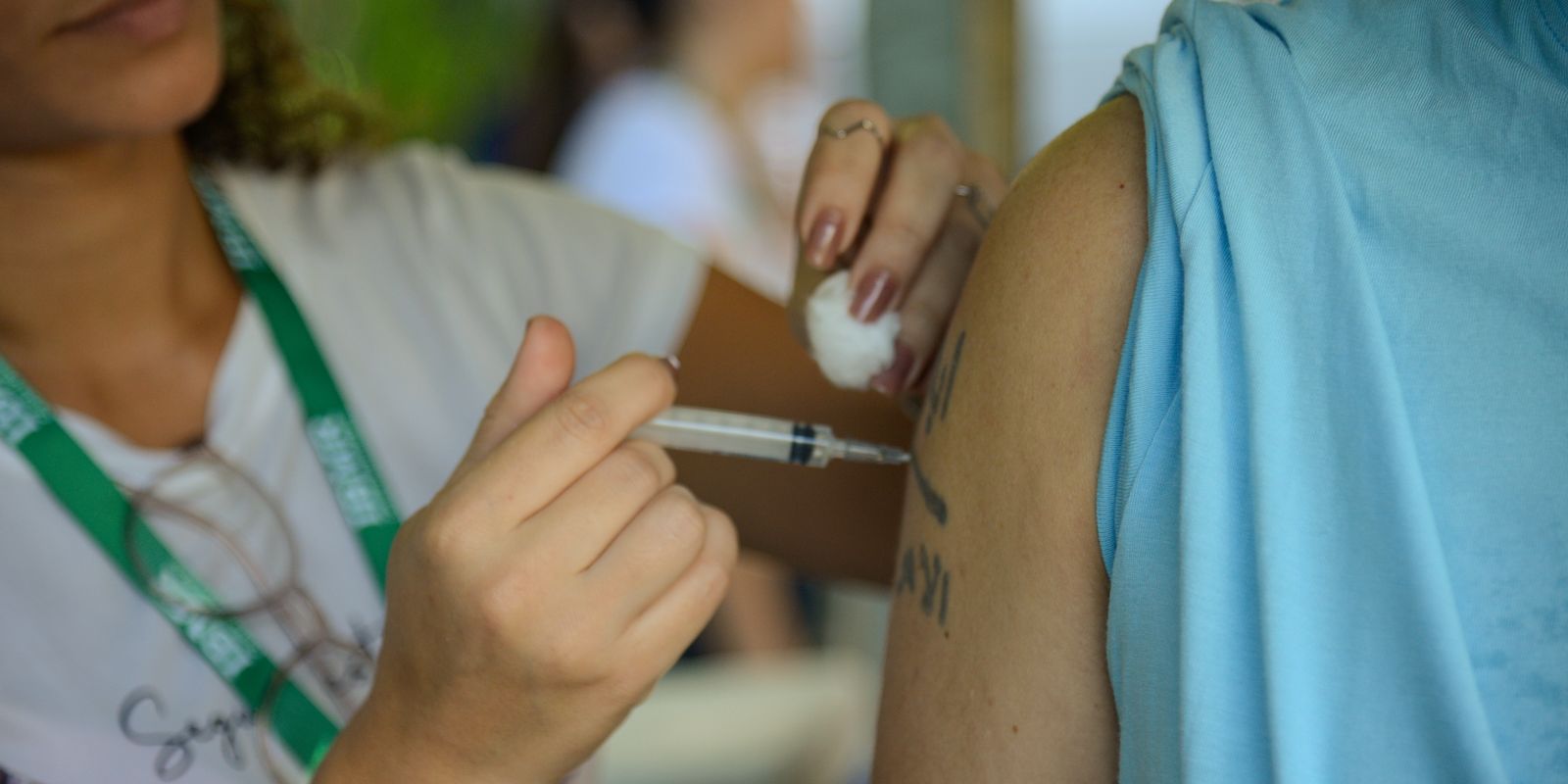
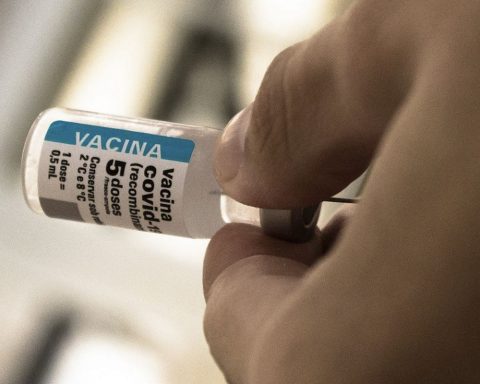
The Ministry of Health opened on Wednesday (17) a public consultation to discuss the incorporation of the vaccine against Herpes Zoster into the National Immunization Program (PNI). The proposal contemplates elderly 80 years or older, and individuals immunocompromised from 18 years.
Public Consultation No. 78 It will be available until October 6th on the participate + Brazil platform. To date, 75 contributions have been registered. Anyone can send opinions and suggestions on the topic.
To participate, it is necessary to fill the electronic form. Interested parties can send up to two files with suggestions or support documents. Sending personal data, sensitive information or third party materials without authorization is not allowed.
Contributions will be analyzed by the coaching staff, which will decide on the incorporation of the vaccine. Technical reports that underpin the preliminary recommendation of the National Commission for Incorporation of Technologies (Conitec), in the Unified Health System (SUS), are available for reading. The analysis and deliberation of the collegiate can also be conferred in the report released by the Ministry of Health.
>> Follow the channel of Brazil agency on WhatsApp
Herpes-zoster
Herpes-zoster, also known as the coat, is caused by the reactivation of the chickenpox virus (chickenpox) and usually reaches elderly and people with low immunity. The disease causes intense pain, fever, spots and bubbles on the skin, which can evolve into serious complications such as postherpetic neuralgia (NPH)-chronic pain that persists even after the end of the lesions.
Between 2008 and 2024, SUS recorded more than 85,000 outpatient care and 30,000 hospitalizations by herpes-zoster. Between 2007 and 2023, 1,567 deaths were associated with the disease. Most were aged 50 or over.
Treatment in the public system involves drugs to relieve symptoms and, in more severe cases, the use of antivirals such as acyclovir. For NPH, drugs such as amitriptyline, carbamazepine and gel lidocaine are offered.
Incorporation into SUS
The incorporation into the SUS of the recombinant vaccine was a request from the Department of the National Immunization Program, the Secretariat of Health and Environment Surveillance, and the Ministry of Health.
The vaccine contains a varicella-zoster (GE antigen) protein combined with an adjuvant (AS01b), which helps the immune system to recognize and combat the virus. It is administered intramuscularly, in two doses of 0.5 ml, with a two -month interval.
Conitec has evaluated the safety and effectiveness of the vaccine. Studies point to more than 80% efficacy in disease prevention and NPH. The most common adverse events reported were: pain in the application site, tiredness, muscle pain, headache and fever, usually of light to moderate intensity. The immunizer was also considered safe.
The high cost is the main challenge: the estimated investment would be $ 5.2 billion in five years. For Conitec, the conclusion is that the vaccine does not offer a sufficiently significant benefit to justify its cost to SUS.