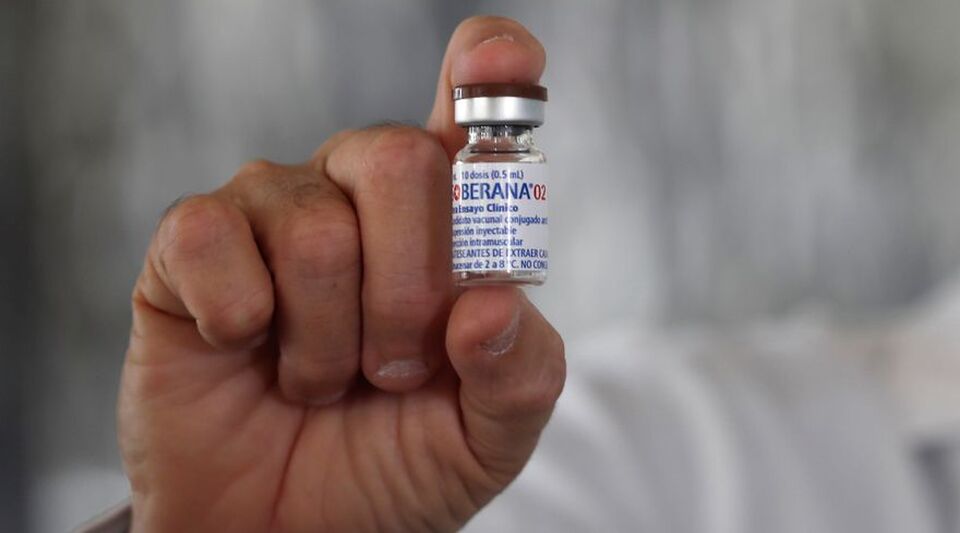
“In Moscow I receive this excellent news. Our Sovereign vaccines continue to present credentials. Congratulations to the Cuban scientists and thanks again for the feat. A hug.” This is how Miguel Díaz-Canel celebrated this Sunday the decision of the Mexican regulator to authorize the emergency use of the Soberana and Soberana Plus vaccines against covid-19, which was made public on Saturday.
The Mexican Federal Commission for the Protection against Sanitary Risks (Cofepris) installed a Regulatory Optimization Committee with the Pan American Health Organization (PAHO), which gave it the status of scientific and regulatory regulator to facilitate cooperation with the other national agencies of the region.
Before her, Pfizer-BioNTech, AstraZeneca, CanSino, Sputnik V, Sinovac, Covaxin, Janssen, Moderna and Sinopharm were also validated.
This is the second Cuban vaccine approved by the Mexican agency, after in December authorized the emergency use of Abdala. Before her, Pfizer-BioNTech, AstraZeneca, CanSino, Sputnik V, Sinovac, Covaxin, Janssen, Moderna and Sinopharm were also validated.
The Soberana and Soberana Plus certification process began more than a year ago in Mexico, and in November 2021 the National Committee for Science and Technology and Innovation in Health (Conacyt) issued its favorable opinion.
The process was stopped, however, until this September, when the Committee for New Molecules (CMN) endorsed the results after the favorable technical opinion of the experts.
“After integrating the opinion of the CMN and entering the authorization request for emergency use before Cofepris, personnel specialized in vaccines analyzed the files, certifying that the biological meets the quality, safety, and efficacy requirements necessary to be applied,” said the Commission in a statement.
The endorsement of the World Health Organization (WHO), however, continues to come neither for Abdala nor for Soberana, without the official press having mentioned the issue again.
The files of both were submitted to the WHO in April this year, with a long delay that the authorities attributed, in the case of Abdala, to changes in the production site.
“The small delay that we have had in our strategy of presenting it to the WHO has been due to an internal element of ours, that we have decided to move to a new (production) plant and that this is the one that undergoes a process” of prequalification, said then the director of BioCubaFarma, Eduardo Martínez Díaz.
The candidate’s status changed to having their documentation accepted and, currently, the process is “underway”, without concluding. Nothing else has been known about Soberana
Martinez said the move to a new complex was “in the process of being adjusted and put into operation.” “That leads to a delay in documentation” and “is one of the elements that has slowed the process down for us,” he added. The candidate’s status began to have his documentation accepted and, currently, the process is “underway”, without concluding. Nothing more has been known about Soberana.
Mexico, however, has continued to acquire the vaccines with the endorsement of its regulator. last septemberthe Government reported the acquisition of nine million doses of Abdala to apply to 3,000,000 children between 5 and 11 years of age, as reported at the time by the Undersecretary for Prevention and Health Promotion, Hugo López-Gatell.
Other countries that have bought the vaccines are Iran, co-producer of Soberana (known as PastoCorona in that country), Venezuela, Nicaragua and Belarus. None of them has revealed the unit cost for which they have been made with the doses, although the Nicaraguan newspaper Confidential, holds which in his country was seven dollars. For the calculation, it takes as a reference the amount of a loan requested by the Ortega government from the World Bank for the payment to Havana, although there is no breakdown that allows verifying each concept of the credit.
________________________
Collaborate with our work:
The team of 14ymedio He is committed to doing serious journalism that reflects the reality of deep Cuba. Thank you for accompanying us on this long road. We invite you to continue supporting us, but this time becoming a member of our newspaper. Together we can continue transforming journalism in Cuba.