Editorial: RADIO PANAMA
The National Pharmacovigilance Center of the National Directorate of Pharmacy and Drugs of the Ministry of Health, following up on the alerts in this case from the European Medicines Agency (EMA), to withdraw the marketing authorizations for the anti-obesity drugs of Amfepramona , informs that the sanitary registration of this product is suspended in Panama.
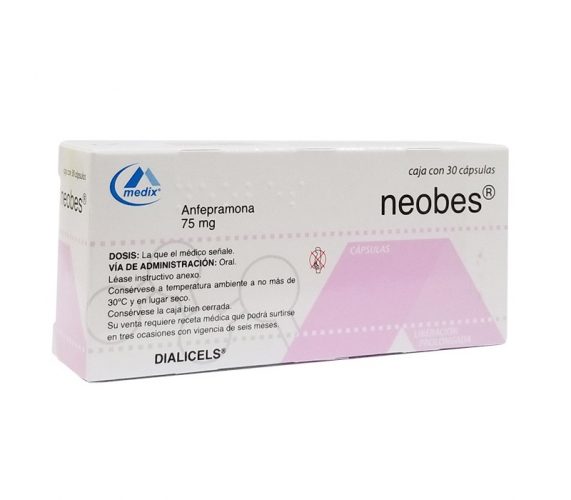
According to MINSA, currently in the National Directorate of Pharmacy and Drugs, the following product with the active ingredient Amfepramone is registered under the trade name Neobes 75mg extended-release capsules, manufactured by the Productos Medix SA de CV laboratory, Mexico with sanitary registration 82955.
To date, the National Pharmacovigilance Center (CNFV) has only received one (1) report of a suspected adverse reaction to a drug associated with the active ingredient Amfepramone, in which strong intestinal colic was reported.
The recommendations of the MINSA through the National Center for Pharmacovigilance is to suspend all current health registrations and the process of renewing and obtaining the health registration of medicines, whose active ingredient is Amfepramone in all its pharmaceutical forms due to the appearance of cases of increased of blood pressure in the arteries of the lungs, heart disease, dependence, psychiatric problems and harm to the fetus.
Patients being treated with Amfepramone should contact their doctor to discuss which treatment would be suitable for them.
The National Center for Pharmacovigilance indicates that we are monitoring this safety announcement and when more information is available it will be communicated.
On October 27, 2022, the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) confirmed its recommendation to withdraw the marketing authorizations for the anti-obesity medicine of Amfepramone.